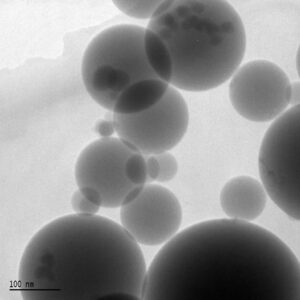
Silica fume, also known as microsilica, is an extremely fine non-crystalline polymorph of silica and is produced in electric arc furnace (EAFs) as a byproduct of silicon and ferrosilicon alloy production. While the first trials of silica fume as an additive in concrete were in the 1950s, at that time much of the silica fume was simply discharge into the atmosphere. It was only with increased environmental legislation in the late 1960s that silica fume began to be collected in bag filters, whereupon manufacturers began to search for use for the material. With the preliminary studies in Norway have shown the potential of silica fume for improving the durability of concrete, a potential market was made.

As just mentioned, silica fume arises as a byproduct of silicon or ferrosilicon alloy formation in an electric arc furnace. In this process, quartz is heated to about 2000oC with the addition of a source of carbon to reduce the quartz to silicon. Small quantities of silicon vapor are released from the furnace whereupon it oxidizes and condenses to form microspheres, which are then collected via fans and bag filters.
Chemical Properties of Silica Fume
- Amorphous
- Silicon dioxide ˃ 85%
- Trace elements depending upon kind of fume
Silica fume particles are incredibly small; with over 95% of the particles being less than 1 µm. size is extremely important for both the chemical and physical gifts of microsilica in concrete.
Amorphous
This term simply means that silica fume is not a crystalline substance. A crystalline material will not dissolve in concrete, which must happen before the material could react. Don’t forget there is a crystalline material in concrete that’s chemically similar to silica fume. That material is sand. While sand is basically silicon dioxide (SiO2) it doesn’t react because of its crystalline nature.
Silicon dioxide
This is the reactive material in silica fume.
Trace elements
Trace components: There may be substances from the silica fume depending upon the metal being produced in the smelter. These substances do not have any impact on the performance of silica fume in concrete.
Physical Properties of Silica Fume
Particle size
The particle size of silica fume is extremely small; with more than 95% of the particles being less than 1µm. particle size is extremely important for both the physical and chemical contributions of silica fume in concrete.
Particles size (typical): ˂ 1 µm
Bulk density
This is just another term for unit weight. The bulk density of the as-produced fume depends upon the metal being made in the furnace & upon how the furnace is operated. Because the bulk density of the as-produced silica fume is usually very low, it is not very economical to transport it for long distances.
Bulk density:
Undensified Silica Fume = 130-430 Kg/m3
Densified Silica Fume = 480-720 Kg/m3
Specific gravity
Specific gravity is a relative number that tells how silica fume compares to water, which includes a particular gravity of 1.0. Silica fume specific gravity is 2.2, which is somewhat lighter than Portland cement, which includes a particular gravity of 3.15. Adding silica fume to the concrete mixture will not density the concrete in terms of increasing the density of the concrete.
This expression means that silica fume is not a substance that is crystalline. The material will not dissolve before the substance could react in concrete, which must occur. Don’t forget that there is a crystalline material in concrete that’s chemically similar to silica fume. That substance is sand, while sand is basically silicon dioxide (SiO2) it does not respond because of its crystalline nature.
Specific Gravity 2.2
Specific surface
The particular surface is the surface area of a given mass of a substance. The surface region is large because the particles of silica fumes are small. We also know that water demand increases for sand as the particles become smaller; the same happens for silica fume. This simple fact is why it is necessary to utilize silica fume in combination. Specific surface determinations based on air-permeability testing or analysis are meaningless for silica fume. This is only another term for component weight. The density of the fume depends upon the alloy being made in the furnace and upon the furnace is operated.
Specific surface area of silica fume 15000-30000 m2/kg
| Chemical Property | Portland Cement | Class F Fly Ash | Class C Fly Ash | Slag Cement | Silica Fume |
| SiO2 Content, % | 21 | 52 | 35 | 35 | 85 to 97 |
| Al2O3 Content, % | 5 | 23 | 18 | 12 | – |
| Fe2O3 Content, % | 3 | 11 | 6 | 1 | – |
| CaO Content | 62 | 5 | 21 | 40 | ˂ 1 |
| Fineness as Surface Area m2/kg | 370 | 420 | 420 | 400 | 15000 to 30000 |
| Specific Gravity | 3.15 | 2.38 | 2.65 | 2.94 | 2.22 |
| General Use in Concrete | As a Primary binder | As a Cement replacement | As a Cement replacement | As a Cement replacement | Property enhancer |